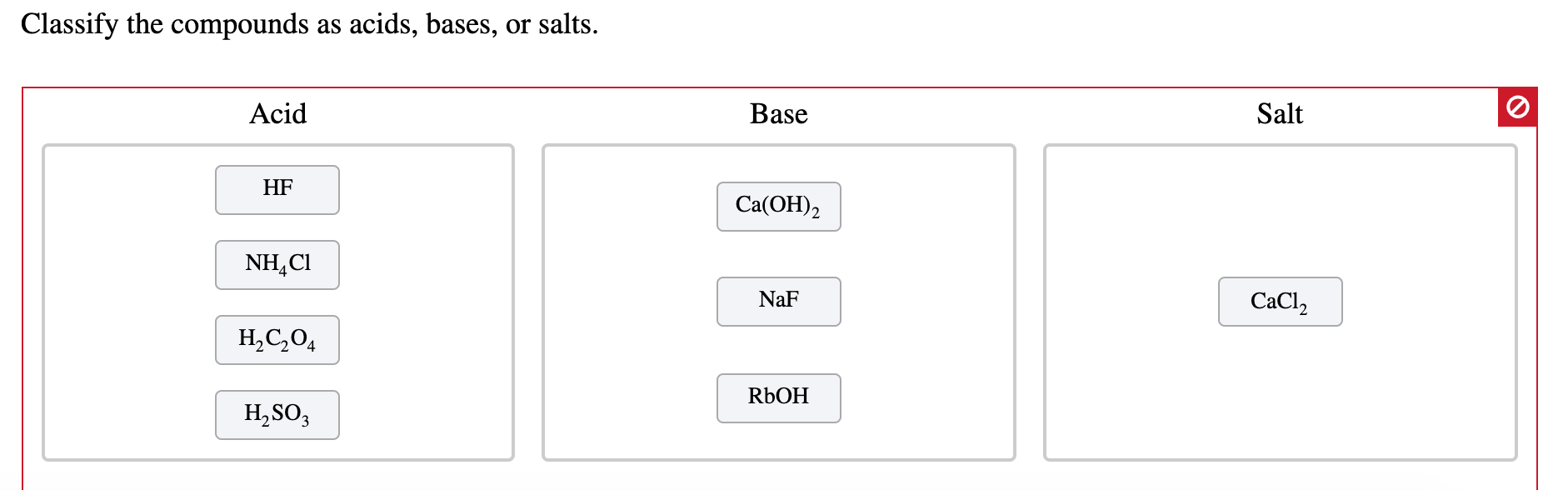
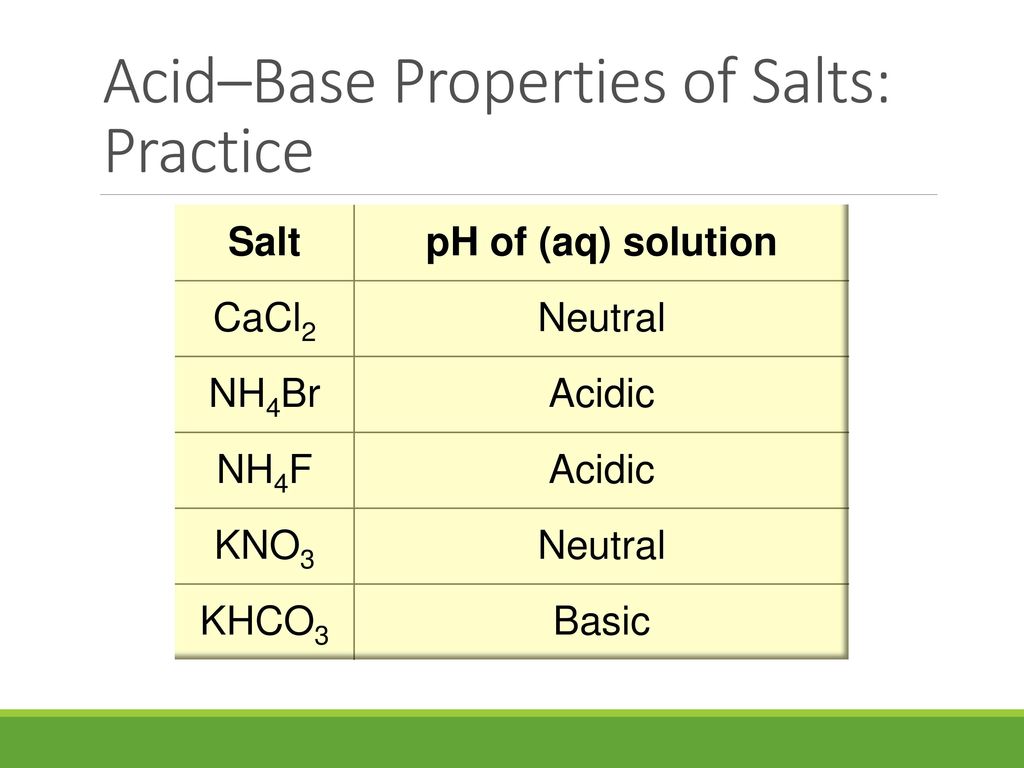
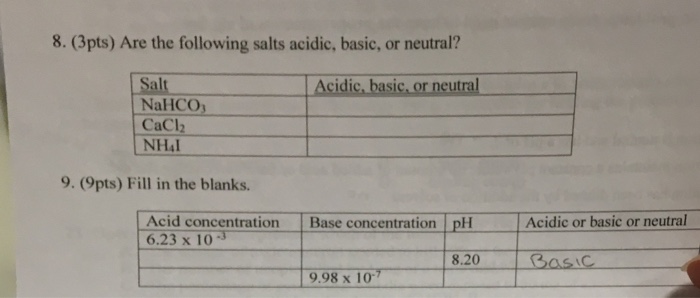
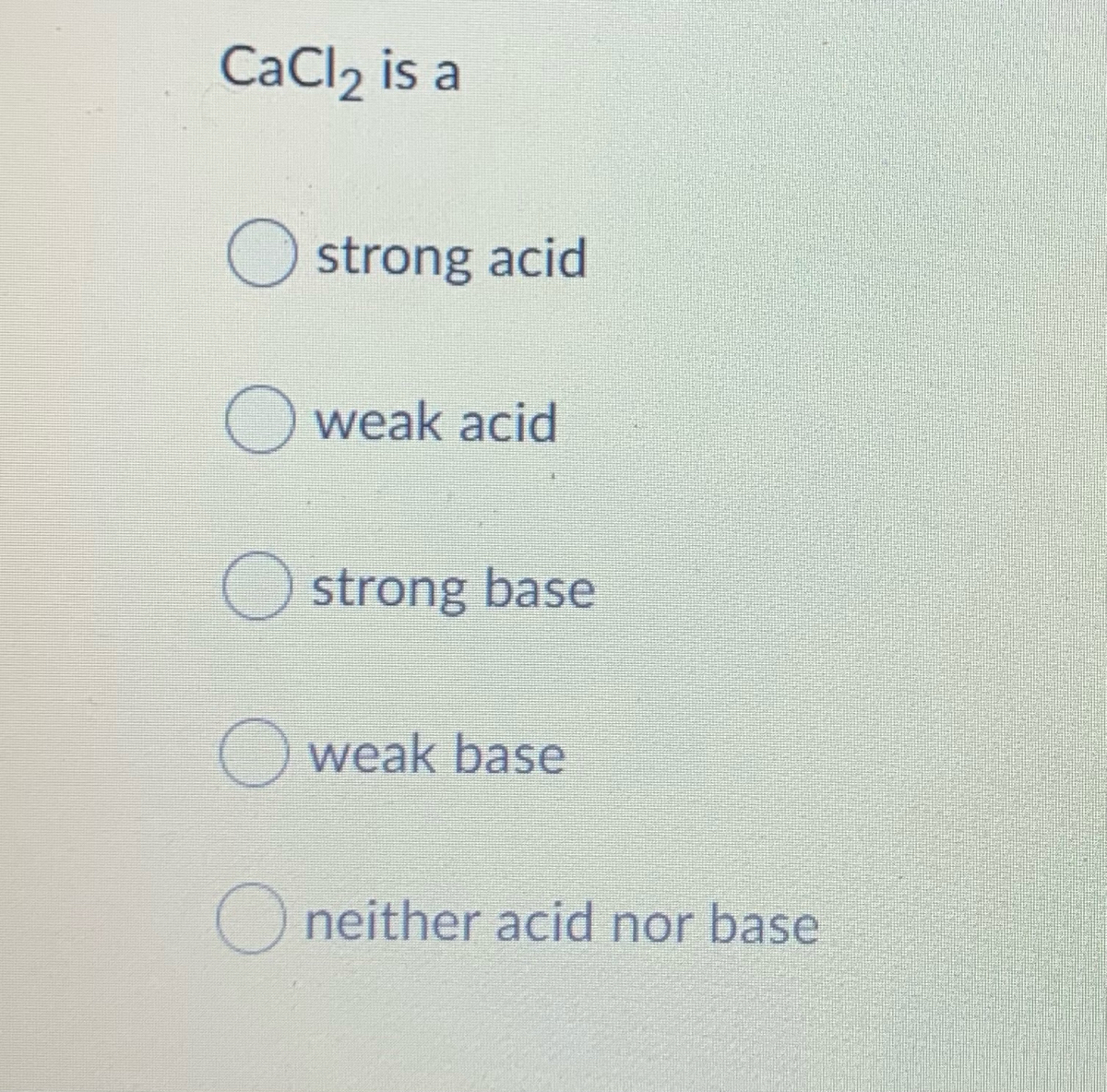
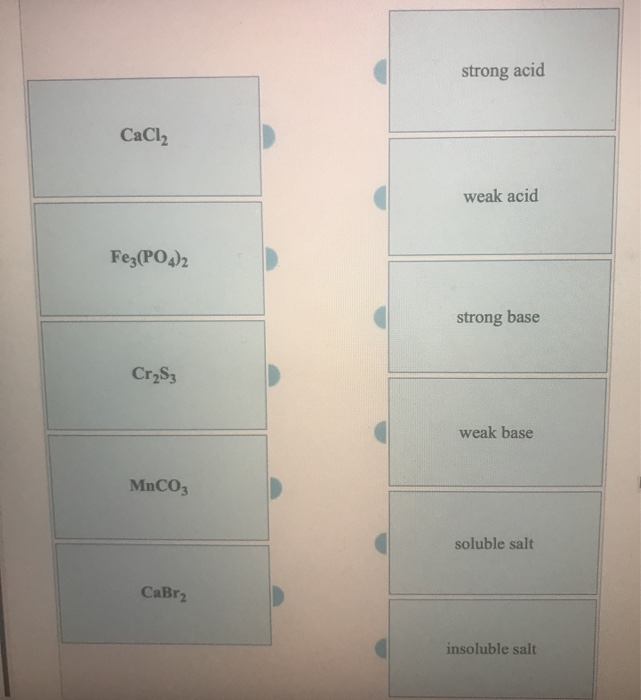
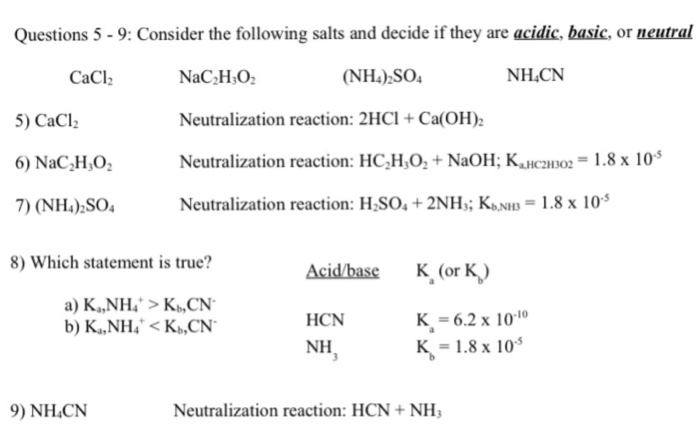
SOLVED: Classify the compounds as acids, bases, or salts. Which are Acids? Which are Bases? Which are Salts? Answer Bank LiCl KOH HBr Ba(OH)2 CaCl2 H2SO3 NH4NO3 H2C2O4

Name the acids and bases from which the following salts may be obtained. (i) Potassium sulphate (ii) Calcium chloride

Mean values of pH in CaCl2, saturation per base and potential acidity... | Download Scientific Diagram

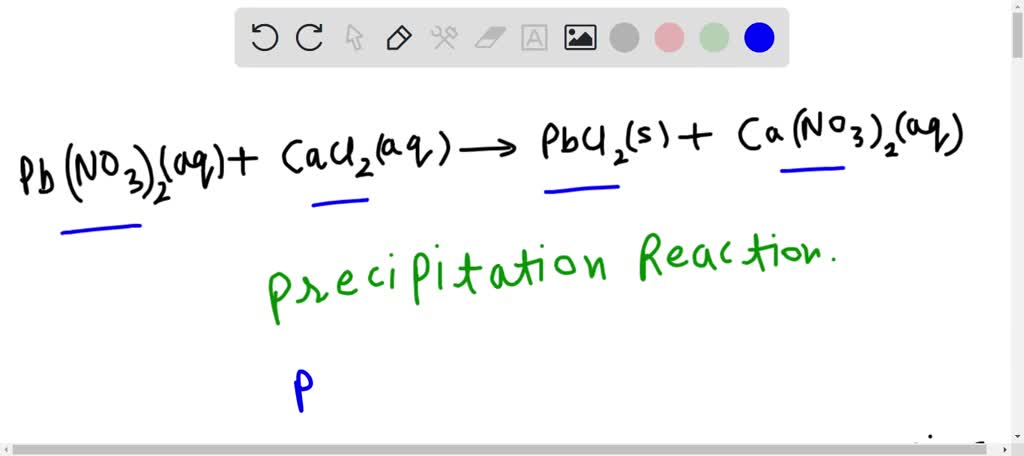
Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

Mean values of pH in CaCl2, saturation per base and potential acidity... | Download Scientific Diagram

Pyrohydrolysis of CaCl2 Waste for the Recovery of HCl Acid upon the Synergistic Effects from MgCl2 and Silica | ACS Sustainable Chemistry & Engineering

organic chemistry - What is role of copper powder, calcium chloride and cuprous chloride in the SN1 reaction of hydrochloric acid with propargylic alcohol? - Chemistry Stack Exchange
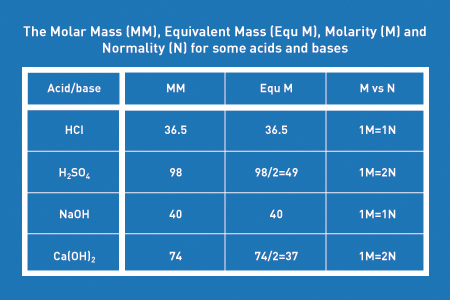







![HCl Gas from conc. HCl(aq) and CaCl2 - [www.rhodium.ws] HCl Gas from conc. HCl(aq) and CaCl2 - [www.rhodium.ws]](https://erowid.org/archive/rhodium/chemistry/equipment/pictures/calcium.hcl.gen.fig.gif)