Exam 3 Practice Problems - Sample Problems (Acid/Base and Solubility) CHM Determine if the following - Studocu
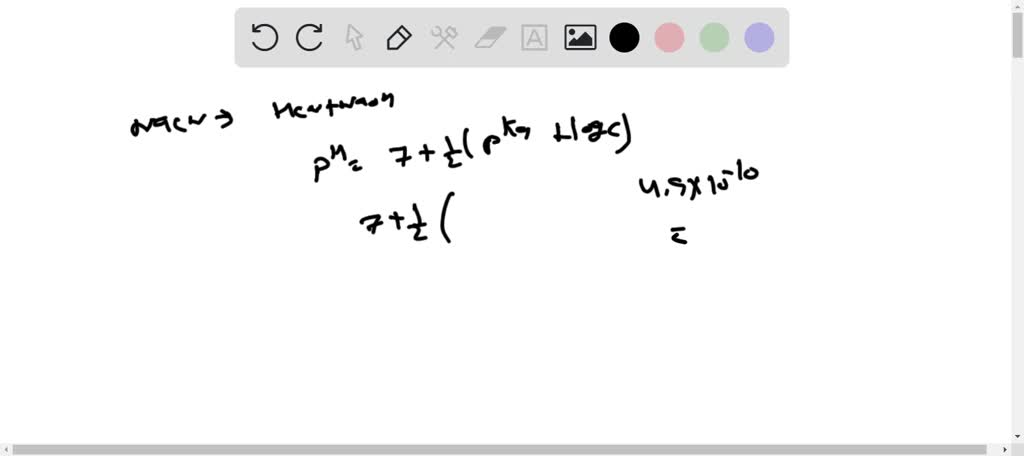
SOLVED: The weak acid hydrocyanic acid, HCN, and the strong base sodium hydroxide react to form the salt sodium cyanide, NaCN. Given that the value of Ka for hydrocyanic acid is 4.90×10−10,
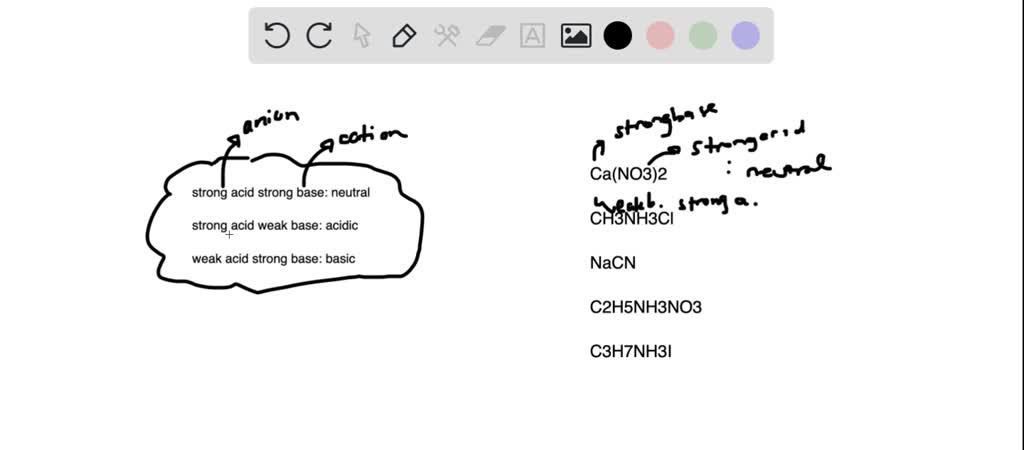
SOLVED: Consider 0.25 M solutions of the following salts. For each salt, indicate whether the solution is acidic, basic, or neutral. Ca(NO3)2 CH3NH3Cl NaCN C2H5NH3NO3 C3H7NH3I

Which pair of compounds will form a buffer in aqueous solution? NaCN and KCN HCl and NaOH NaCN - Home Work Help - Learn CBSE Forum